The New Standard
of Truth for the
Bio-Economy
The next generation of supply chain management blockchain-enabled software for tracking, tracing, and verification of biologics from source to consumer/patient.
1. THE need
uncertainty is a luxury
we cant afford.
eliminating the "black box" of biologics
TRUST IS BROKEN
- The Chain of Identity Failure:
Current systems cannot definitively verify the true origin, content, quality, processing, and handling of valuable biologic material. Lack of reliable provenance data leads to increased risk of errors, fraud, and inefficient validation processes at different points of the product lifecycle.
- Safety Risks and Counterfeiting:
The inability to track assets end-to-end creates vulnerabilities for counterfeit or compromised products, drugs, and therapies to enter the market. Once falsified medicines enter the supply chain, they endanger lives across the globe, and erode public trust . This international threat is magnified by the growing online market for falsified medicines. The World Health Organization defines falsiified medicines as, "medical products that deliberately/fraudulently misrepresent their identity or source."
- Production and Handling:
Critical data regarding safety, temperature, expiry, handling protocols, and chain-of-custody is often siloed or lost. Stakeholders lack real-time visibility into the conditions the asset endured during transit and processing.
2. The Solution:
the Biotrace engine
the biotrust, Biopassport, biotether
& intelligaurd ai
Next-generation bioverification
the BioTRUST
immutable blockchain
We utilize a decentralized ledger to create an unalterable, validated history of every asset's birth, handling, and disposition, ensuring data integrity across fragmented ecosystems and multiple geographies, from lab to bedside.

The biopassort
dynamic Encryption
Manufacturers cryptographically prove the origin and integrity of a product to a regulator, hospital, or consumer without revealing sensitive data. We allow the "truth" of the data to be shared while keeping the data itself mathematically sealed. Our dynamic Trust Seal makes is mathematically impossible for counterfeiters to clone packaging or reuse serial numbers.

The IntelliGuard
Artificial Intelligence
IntelliGuard Ai Autonomous Enforcement secures safety of sensitive data before and after the data is entered into the BioTrace Trust. IntelliGuard actively patrols and audits the Trust for fraud, duplicates, counterfeiting, illogical volumes, safety expirations and risks, spoilage, and cross-border regulatory complexity for compliance.
4. The Biotether
Token Economy
BioTether is the dedicated utility currency designed to fund the operational costs of the high volume track, trace, and verify system, acting as the fuel that powers the computational and economic costs of each ledger entry and verification. As the network and volume of biologics scales, so does the demand for the token. The token's value is therefore intrinsically linked to the volume of real-world biologic assets produced and secured and verified on the system.
1. Collection (Hospital)
Medical data enters the system through innovation and advanced digital health technology.

2. PROCESSING (Lab)
Medical data enters the system through innovation and advanced digital health technology.
3. PACKAGING (Head-off)
Medical data enters the system through innovation and advanced digital health technology.

4. MANUFACTURING (Transformation)
Medical data enters the system through innovation and advanced digital health technology.
5. DISTRIBUTION (Retail)
Medical data enters the system through innovation and advanced digital health technology.

1. Collection (Hospital)
Medical data enters the system through innovation and advanced digital health technology.

2. PROCESSING (Lab)
Medical data enters the system through innovation and advanced digital health technology.

3. PACKAGING (Head-off)
Medical data enters the system through innovation and advanced digital health technology.

4. MANUFACTURING (Transformation)
Medical data enters the system through innovation and advanced digital health technology.

5. DISTRIBUTION (Retail)
Medical data enters the system through innovation and advanced digital health technology.

1. Collection
Secure Origin Capture: The chain of identity begins here. The source meta data enters the system, like a birth certificate, and is cryptographically hashed onto the ledger, creating the biological asset's digital twin. A BioPassport is created for the harvested or created source material.

2. PROCESSING
Immutable Lab Records: Processing protocols, quality analysis, cell counts, and viability test results are automatically logged in the BioPassport and BioTrace Trust . IntelliGuard audits this data against regulatory baselines to certify quality before the asset moves forward.
3. PACKAGING
Smart Serialization: The biologic is secured in BioTrace enabled packaging. A unique serialiozed BioPassport is minted and attached, linking the biomaterial to its blockchain Trust record for granular tracking.

4. MANUFACTUring
Genealogy Mapping: As raw materials are transformed into finished therapeutics or consumer packaged goods, the system maps the "parent "inputs" to the "child" products, maintaining a complete, unbreakable family tree of the biologic's creation.

5. DISTRIBUTION
Dynamic Updates: Logistics providers earn BioTether by providing real-time data to the Trust and BioPassport.
6. RetaiL
Last Mile Authenticqation: Pharmacies, clinics, and retailers utilize the BioTrace Trust Portal to instantly verify the entire pedigree of the drug, confirming it hasn't been diverted or substituted.
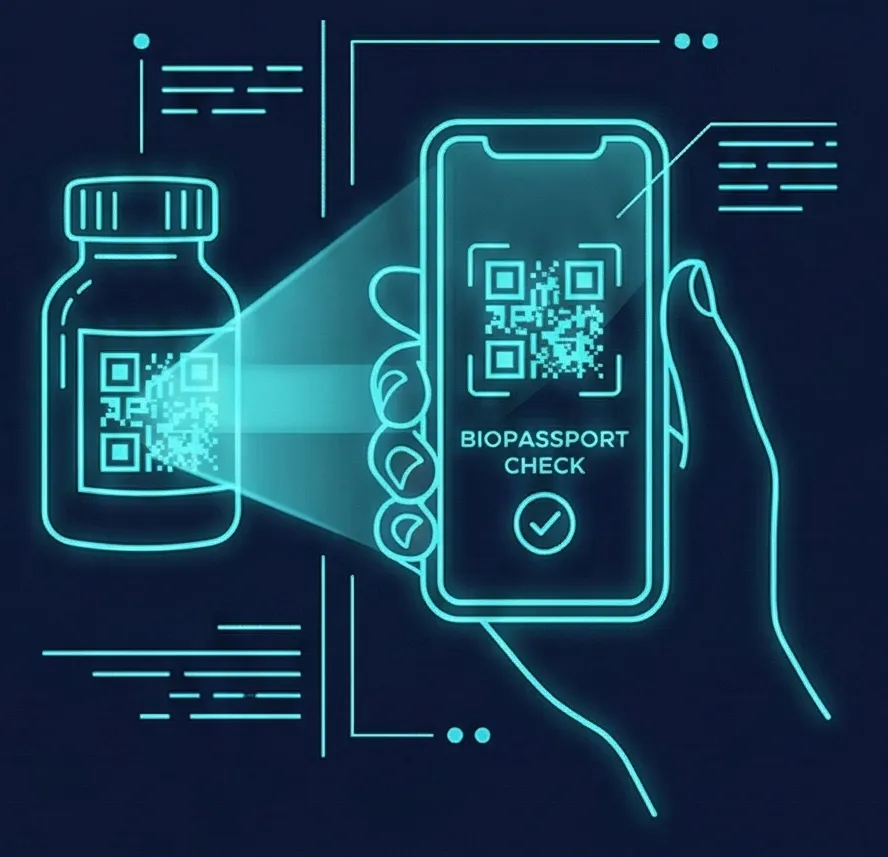
7. Consumption
The Ultimate Proof: Consumer scans the final product or treatment to receive a BioPassport with detailed confirmation of authenticity, safety, and handled history, restoring trust in the products and biology consumers depend on.
5. universal value proposition
illuminating the lifecycle

Government Compliance Node
Access aggregated and immutable insights into the BioTrust chain, audit logs, traceability reports, industry-wide safety standards, and supply chain integrity.

Anti-Counterfeiting
& Automated Compliance
Eliminate manual auditing with real-time, blockchain-backed reports that satisfy global requirements for provenance and supply-chain integrity.

Rapid Crisis Response
Instantly execute targeted recalls by tracing affected batches back to the source or forward to the specific patient in seconds, not weeks.

Fraud Prevention & Liability Reduction
Stochiometric Smart Contracts remove human error and enforce the dependency between raw material and finished goods, eliminating phantom production and volume fraud.

Brand Protection
& Premium Asset Valuation
Differentiate your biologics with a verified Digital Birth Certificate, Twin, and BioPassport, commanding higher market value through guaranteed quality and zero-fault provenance.

Global Regulatory
Pre-Clearance
Ai enabled GateKeeper ensures dynamic requirements of host country are met at each step of the lifecycle, preventing customs seizures and destruction of valuable materials, and increasing comliance speed, efficeincy, and accuracy.

Zero-Fault Liability
Use immutable sensor logs to prove handling integrity, protecting your organization from liability claims due to upstream or downstream handling errors.

Active Surveillance
ctive Cold-Chain Surveillance Real-time GPS and thermal alerts ensure that excursions are caught instantly, preventing the delivery of compromised goods and reducing waste.

Cryptographic Handoffs
Cryptographic Handoffs Streamline transfers between facilities with digital signatures that ensure the "Chain of Identity" remains unbroken at every mile.

Bedside Safety Verification
Instantly verify the entire pedigree and safety history of a biologic before administration, ensuring 100% patient safety and professional peace of mind.

Automated Quality Assurance
Automated Quality Assurance Link BioPassport data directly to internal records to automate regulatory reporting and eliminate the risk of manual data entry errors.

Inventory Intelligence
Know exactly what is in your inventory, its exact origin, and its precise expiration with forensic-level accuracy that prevents stock-outs or spoilage.

Radical Traceability
Consumers can view and interact with a trusted BioPassport record of a products source, geographic origin, quality, testing data, processing, transit history, safety and other real-time data.

The End of Counterfeits
Protect your health with unforgeable authenticity. Rest easy knowing that your high-stakes therapies, treatments, skincare, supplements, or drugs are 100% authentic, verified by a secure blockchain ledger that prevents fraudulent copies.

Dynamic Privacy
You own your data. Advanced zero-knowledge encryption ensures you control who sees your biological history and outcomes, keeping your identity 100% private.
6. THE Biotrace
technical team
Gavin Sathianathan
Chief Executive Officer & Director
Gavin Sathianathan is an entrepreneur, operator and digital strategist with 20+ years of experience institutionalizing innovation at the intersection of consumer data, life sciences, and frontier growth sectors. He is a recognized specialist in digital transformation, having spent 15 years at the vanguard of the corporate world where he bridged the gap between complex data ecosystems and commercial execution.
Mr. Sathianathan spearheaded global digital transformation initiatives for Meta (Facebook), Bain, and Tesco. During his tenure in the retail and media sectors, he mastered the art of leveraging consumer data at scale—expertise he later pivoted to the healthcare industry to solve systemic inefficiencies in clinical research. He co-founded Oxford Cannabinoid Technologies, a London-based pharmaceutical developer, where he navigated the complex regulatory and capital hurdles required to develop licensed medicines.
Mr. Sathianathan is the driving force behind Research Smart, an AI-driven, end-to-end research platform designed to disrupt the traditional clinical trial model. By leveraging intuitive consumer design and mobile-first technology, the platform democratizes access to research for underrepresented cohorts, including BAME populations and the rare disease community. His work focuses on unlocking value in botanical medicines and generic drug repurposing, through a digital framework that generates personalized, predictive insights for individual patient therapies.
Mr. Sathianathan holds a degree in Chemical Engineering from Imperial College London and an MBA from Harvard Business School.
Mr. Sathianathan spearheaded global digital transformation initiatives for Meta (Facebook), Bain, and Tesco. During his tenure in the retail and media sectors, he mastered the art of leveraging consumer data at scale—expertise he later pivoted to the healthcare industry to solve systemic inefficiencies in clinical research. He co-founded Oxford Cannabinoid Technologies, a London-based pharmaceutical developer, where he navigated the complex regulatory and capital hurdles required to develop licensed medicines.
Mr. Sathianathan is the driving force behind Research Smart, an AI-driven, end-to-end research platform designed to disrupt the traditional clinical trial model. By leveraging intuitive consumer design and mobile-first technology, the platform democratizes access to research for underrepresented cohorts, including BAME populations and the rare disease community. His work focuses on unlocking value in botanical medicines and generic drug repurposing, through a digital framework that generates personalized, predictive insights for individual patient therapies.
Mr. Sathianathan holds a degree in Chemical Engineering from Imperial College London and an MBA from Harvard Business School.
David Gobaud
Co-Founder & Inventor
David Gobaud is an entrepreneur, operator, and strategic investor with a proven track record of building and scaling disruptive platforms at the intersection of fintech, blockchain, supply chain management, and global regulatory frameworks. He was the CEO and founder of Passfolio, a premier financial services company that increased global financial democratization by providing seamless access to U.S. stock trading, cryptocurrency, and banking services to users worldwide.
Mr. Gobaud led the successful acquisition of Passfolio by one of the world’s 20 largest banks, a landmark exit in the fintech sector. A pioneer in the decentralized economy, he also founded Mobius, a blockchain enterprise that completed a $39M token sale for its decentralized application (DApp) store. His entrepreneurial portfolio further includes the creation of Pluto Mail, an ephemeral email service, and Yoshi, a Y Combinator-backed automated fuel delivery company.
Beyond his operational achievements, Mr. Gobaud is a recognized expert in technology law and policy. As a Mitchell Scholar, his research at the intersection of innovation and liability—specifically regarding autonomous vehicles—was conducted at the Stanford Center for Internet and Society. This work established him as a strategic thinker capable of navigating the complex legal landscapes of frontier technologies.
Mr. Gobaud holds an LLM in Corporate Governance from Queen’s University, Belfast. He completed his legal and technical education with an undergraduate degree in Computer Science from Stanford University and a Juris Doctor (JD) from Harvard Law School.
Mr. Gobaud led the successful acquisition of Passfolio by one of the world’s 20 largest banks, a landmark exit in the fintech sector. A pioneer in the decentralized economy, he also founded Mobius, a blockchain enterprise that completed a $39M token sale for its decentralized application (DApp) store. His entrepreneurial portfolio further includes the creation of Pluto Mail, an ephemeral email service, and Yoshi, a Y Combinator-backed automated fuel delivery company.
Beyond his operational achievements, Mr. Gobaud is a recognized expert in technology law and policy. As a Mitchell Scholar, his research at the intersection of innovation and liability—specifically regarding autonomous vehicles—was conducted at the Stanford Center for Internet and Society. This work established him as a strategic thinker capable of navigating the complex legal landscapes of frontier technologies.
Mr. Gobaud holds an LLM in Corporate Governance from Queen’s University, Belfast. He completed his legal and technical education with an undergraduate degree in Computer Science from Stanford University and a Juris Doctor (JD) from Harvard Law School.

